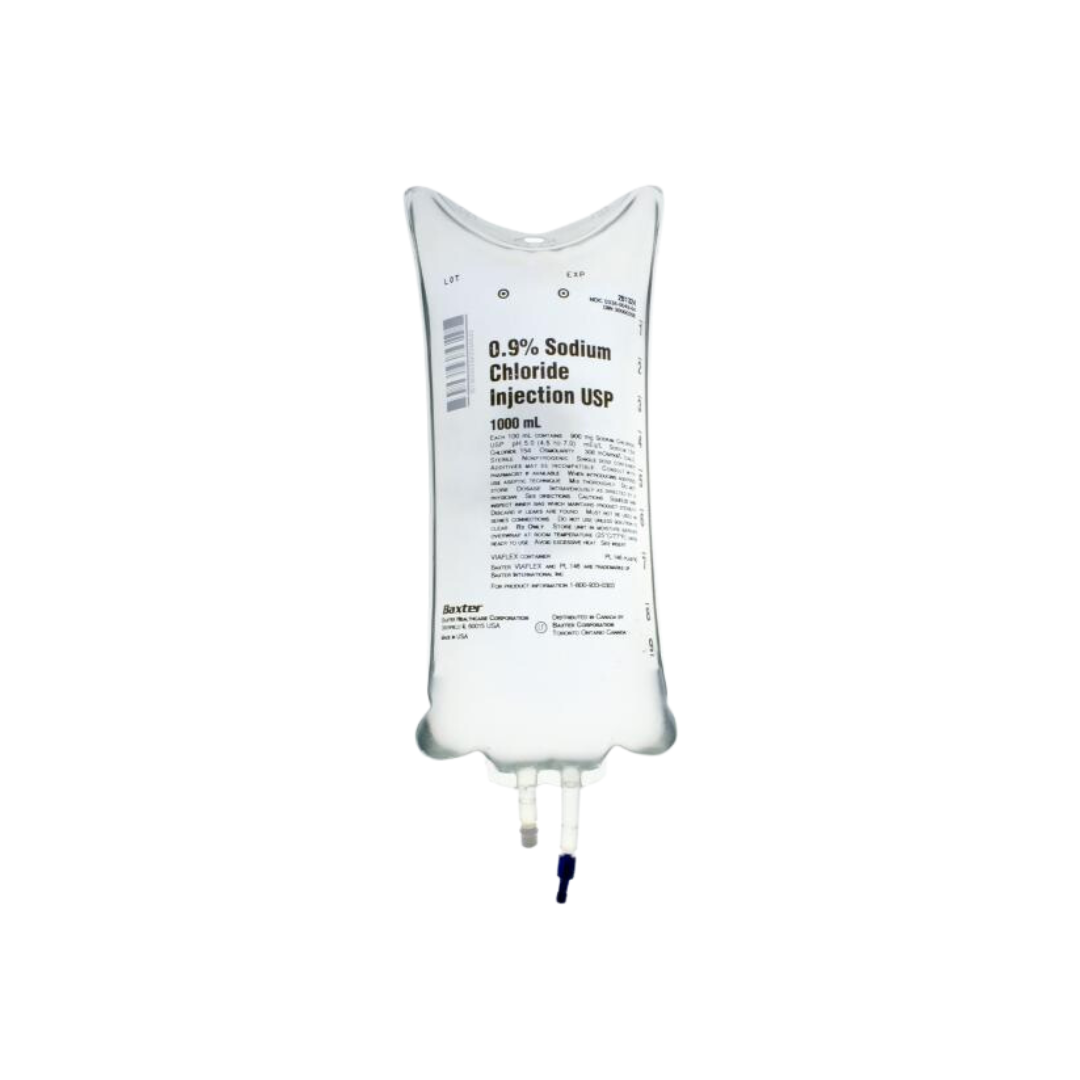
Baxter's 0.9% Sodium Chloride Injection Bags, USP system is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered or liquid (up to 10 mL) drug vial.
Specifications
- Not Made with Natural Rubber Latex
- Container Type: VIAFLEX
- PVC: Contains PVC
- DEHP: Contains DEHP
- Volume: 1000mL, 500mL, or 250mL
- Sodium Concentration: 0.9%
- Sodium: 154 mEq/L
- Chloride: 154 mEq/L
- Osmolarity (mOsmol/L): 308
- Specific Gravity: 1.01
- pH: 5
- Fill Range Volume (mL): 1030 - 1070
- Shelf Life from manufacturer: 18 months
- Contains Preservative: No
- Storage Recommendations: Store at room temperature (25°C/77°F); brief exposure up to 40°C/104°F does not adversely affect the product.